Celltrion says its COVID-19 treatment cuts recovery time, chance of severe cases
By Kim Han-joo
SEOUL, Jan. 13 (Yonhap) -- South Korean pharmaceutical giant Celltrion Inc. said Wednesday that its COVID-19 treatment candidate reduces recovery time and the chance of a severe case developing.
It was making public clinical data on the second-phase trial study of the CT-P59, an anti-COVID-19 monoclonal antibody treatment candidate. The firm earlier submitted an application for conditional marketing authorization of the product to the Korean Ministry of Food and Drug Safety (KMFDS).
The company conducted the trial on 327 patients with mild-to-moderate symptoms of the novel coronavirus to evaluate the efficacy and safety of CT-P59, also known as Regdanvimab.
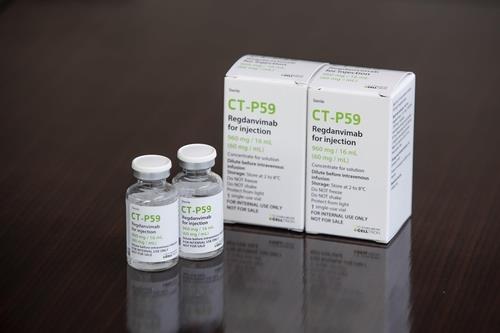
This file image, provided by South Korea's pharmaceutical giant Celltrion Inc. on Dec. 29, 2020, shows the company's anti-COVID-19 monoclonal antibody treatment. (PHOTO NOT FOR SALE) (Yonhap)
Celltrion said CT-P59 decreased the rate of COVID-19 patients developing severe cases, which require hospital care, by 54 percent.
Also, it cut recovery time by more than three days, the company said, adding there has been no serious adverse drug reaction among the patients who took part in the clinical trials.
The KMFDS is reviewing its safety and efficacy, a crucial procedure for the manufacturing and sales of South Korea's first homegrown COVID-19 treatment.
The company also plans to submit an application for Emergency Use Authorization (EUA) with the U.S. Food and Drug Administration and for Conditional Marketing Authorization to the European Medicines Agency (EMA) in the coming months.
The company is also working to increase its manufacturing capabilities to meet the current global and domestic demand for the treatment CT-P59.
khj@yna.co.kr
(END)
-
 S. Korea marks 30th anniv. of Korean Pavilion at Venice Biennale with contemporary art
S. Korea marks 30th anniv. of Korean Pavilion at Venice Biennale with contemporary art -
 Artist Lee Bae captures ethereal Korean aesthetics at Venice Biennale
Artist Lee Bae captures ethereal Korean aesthetics at Venice Biennale -
 Ateez member Yunho throws first pitch at MLB match between Dodgers, Mets
Ateez member Yunho throws first pitch at MLB match between Dodgers, Mets -
 Gov't likely to accept university chiefs' request to lower med school enrollment quota
Gov't likely to accept university chiefs' request to lower med school enrollment quota -
 S. Korea supports resolution backing U.N. membership of Palestine
S. Korea supports resolution backing U.N. membership of Palestine
-
 Artist Lee Bae captures ethereal Korean aesthetics at Venice Biennale
Artist Lee Bae captures ethereal Korean aesthetics at Venice Biennale -
 S. Korea marks 30th anniv. of Korean Pavilion at Venice Biennale with contemporary art
S. Korea marks 30th anniv. of Korean Pavilion at Venice Biennale with contemporary art -
 Gov't likely to accept university chiefs' request to lower med school enrollment quota
Gov't likely to accept university chiefs' request to lower med school enrollment quota -
 Yoon's approval rating sinks to lowest point since taking office
Yoon's approval rating sinks to lowest point since taking office -
 (LEAD) Yoon proposes first-ever meeting with opposition leader
(LEAD) Yoon proposes first-ever meeting with opposition leader
-
 (LEAD) N. Korea sends economic delegation to Iran amid suspected military cooperation
(LEAD) N. Korea sends economic delegation to Iran amid suspected military cooperation -
 N.K. leader's sister slams joint S. Korea-U.S. military drills
N.K. leader's sister slams joint S. Korea-U.S. military drills -
 Author of ADOR's 'internal document' refutes allegations of spinoff plot
Author of ADOR's 'internal document' refutes allegations of spinoff plot -
 Yoon says N. Korea must not dare challenge S. Korea
Yoon says N. Korea must not dare challenge S. Korea -
 U.S. will take steps for three-way engagement on nuclear deterrence with S. Korea, Japan: Campbell
U.S. will take steps for three-way engagement on nuclear deterrence with S. Korea, Japan: Campbell





















